- Anti-oxidant/anti-inflamatory nanoparticles to protect the brain from excess electrophysiology and to mitigate damage post-crisis
- Characterization of the didys552 zebrafish mutant line (mutation in snc1lab gene) and set up of an efficacy drug screening assay using reference compounds
- Cell Therapy with GABAergic interneuron precursors for Early Infantil Epileptic Encephalopathies (S. Dravet, S. West y S. Stxbp1)
- Creation of a therapeutic drug monitoring (TDM) unit for the optimization of the Dravet syndrome pharmacological therapy
- Design, synthesis and pharmacological evaluation of new neuroprotective agents oriented to the tratment of Dravet syndrome
- Efecto de campos magnéticos estáticos de intensidad moderada en modelos de epilepsia y síndrome de Dravet
- The effect of beta-caryophyllene treatment in a murine model of Dravet syndrome
- The endocannabinoid system study in Dravet syndrome
- Intrinsic neuronal excitability and spontaneous 1 activity underlie cortical abnormalities upon Nr2f1/COUP-TFI deficiency
- Investigating Epilepsy by Super-resolution Imaging of Synapses and the Extracellular Space in Live Brain Tissue
- Precision Medicine in Dravet Syndrome
- Reactive Neurogenesis and Gliogenesis in a Dravet Syndrome Mouse Model
(Cell-based Therapy for Neuropathologies Lab)
(Cell-based Therapy for Neuropathologies Lab)
Timeline: 2 years.
Project Costs: 100.000€ per model/syndrome
Manuel Álvarez Dolado

OBJECTIVE
To perform a pre-clinical trial for the evaluation of the therapeutic potential of GABAergic interneuron precursor transplants in animal models of Early Infantil Epileptic Encephalopathies (Dravet, West and Stxbp1 Syndromes). We will analyze their safety and effects at electrophysiological, molecular, histological and behavioral levels.
State of the Art
Epilepsy is a chronic condition due to an imbalance between excitation and inhibition that leads to uncontrolled neuronal hyperexcitability. In general, seizures may be due to hyperactivity of excitatory neurons or, conversely, low activity of the interneurons that constitute the inhibitory system, usually due to alterations in GABAergic neurons. Many evidences have established a strong link between GABA and epilepsy. Hence, antiepileptic drugs have the GABAergic system as one of their main target. However, despite the proven efficacy of these drugs, there is a serious refractory issue among epileptic patients. About 30% of epilepsies are untreatable with first line drugs. This figure can reach up to 70% of patients with early childhood epileptic encephalopathies (EIEE). These are characterized by three main diagnostic criteria: medically refractory crisis, diffuse encephalopathy and strong delay in psychomotor development. This classification includes Stxbp1 Syndrome; West Syndrome (WS); and Dravet Syndrome (DS). These conditions are poorly characterized and lack of an effective pharmacological treatment. Therefore, the development of new therapeutic alternatives is urgently needed, given their severe symptoms that lead to significant developmental delay, disability, and high risk of sudden unexpected death of the epilepsy (SUDEP).
In the last few years cell-based therapies have been proposed as a system to modulate local circuit hyperactivity or increase the inhibitory activity in order to treat epilepsy. Since many EIEE are characterized by deficits in interneuron number or their activity, a novel therapeutic approach could be a cell-replacement strategy of the defective GABAergic interneurons. The present project attempts to use MGE-derived GABAergic neuronal progenitors as a new cell-based alternative for EIEE treatment.
For this, we already count with several mouse models of WS, DS and Stxbp1 in our lab. In addition, our group has an extensive experience in the use of these interneuron precursors for cell therapy. We have shown that MGE-derived progenitors differentiate into GABAergic interneurons when transplanted into the normal neonatal mouse brain. These cells migrate long distances (3-4 mm.) integrating widely in the cerebral cortex, hippocampus and striatum (Fig. 1a). They differentiate, acquiring characteristic morphologies and molecular markers of GABAergic interneurons (Fig. 1b-g) and integrate into the cortical and hippocampal circuitry. We have shown that they are electrophysiologically functional, presenting typical action potentials and membrane properties of mature GABAergic interneurons and are able to modify the general inhibitory tone in the grafted zones.
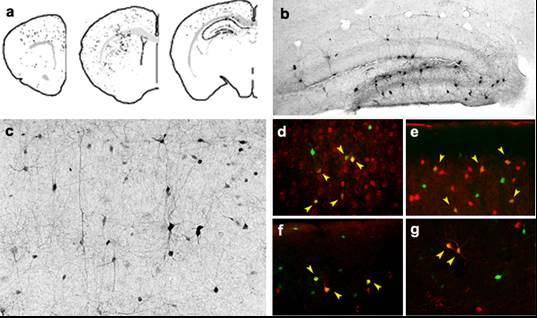
Figure 1: (a) Distribution of MGE-derived cells after a single intracortical injection. GFP+ cells were detected by inmunohistochemistry 1 month after transplantation. Observe the acquisition of mature interneuron morphologies in the hippocampus (b) and cerebral cortex (c). Cells expressed neurotransmitters and specific molecular markers of mature interneurons such a GABA (d), Parvalbumin (e), Somatostatin (f) and NP-Y (g). Arrows show co-localization with GFP.
These characteristics strongly suggest the suitability of these cells for a therapeutic application to treat EIEE. Their naturally occurring differentiation to GABAergic neurons, and their wide migration through the affected areas, would make them ideal to replace the lack of GABAergic neurons observed in this syndromes, or, at least, as a direct source of GABA that may prevent the spasms or seizures. Supporting this hypothesis, we demonstrated that grafts of these precursors replace physically and functionally a specific deficit in hippocampal interneurons that lead to higher susceptibility to seizures in adult mice. Grafts of these precursors present an important anticonvulsant activity, avoiding seizure generalization, and the transplants reduce significantly the mortality rate as consequence of induced seizures. Finally, these cells are extremely stable and safe, since no tumor formation has been reported more than one year after their transplant. Moreover, recently we transplanted these cells in an animal model of Alzheimer disease that presents a deficit in the expression of Nav1.1, as occurs in DS. The results, already published in Neuron, show the rescue of cognitive alterations and brain rhythms in this model after the transplants. The analysis of the EEG wave spectrum indicates a normalization of cerebral rhythmogenesis in these animals, which leads to a reduction in their hyperactive-anxiety behaviors, and the recovery of normal cognitive levels. In addition, preliminary assays in our WS mouse model show the normalization of behavioral symptoms and a significant reduction in the frequency of epileptic spasms.
All these results strongly suggests the suitability of MGE-derived neuronal precursors for cell-based therapies, and opens a new avenue for the treatment of disorders related with deficits in interneurons, as is the case of EIEE.
In summary, the project will allow a further understanding of EIEE and complete pre-clinical trials in animal models to assay a novel therapeutic strategy based on GABAergic neuronal precursors. We take the challenge of developing new treatments for these devastating pathologies that affect children, and have no solution at the moment.
Methods and Phases
To achieve this objective, the project will be divided into 4 phases, each lasting 6 months, in which some milestones will be established to continue with the project. At the end of each phase, follow-up meetings will be set to report results and difficulties. The following milestones will be achieved in each phase:
Phase 1
- To obtain a large colony of mouse models to perform all the trials.
- To characterize at behavioral and EEG levels the mouse model.
- To breed the model and donor mice to perform the neonatal transplant assay during the next phase.
Phase 2
- To perform transplants in neonatal mice.
- To analyze the effects of transplantation at the behavior level.
- To breed the model and donor mice to perform the adult transplant assay during the next phase.
Phase 3
- To analyze the effects of neonatal transplantation at the electrophysiological level (EEG, crisis reduction, wave spectrum).
- Sample collection of the neonatal cohort.
- To perform transplants in adult mice.
- To perform behavioral tests in the adult cohort.
Phase 4
- To analyze the effects of adult transplantation at electrophysiological and behavioral levels.
- Sample collection of the adult cohort and histological and molecular analysis together with the neonatal cohort.
- Global analysis of the project, conclusions and final report.
Personnel hiring
Mouse models breeding
COOPERATIVE ORGANIZATIONS
CABIMER
SPONSORS
Ministerio de Educación y Ciencia, Junta de Andalucía, Fundación Ramón Areces, Apoyo Dravet

